Research
Led by Katherine A. Gallagher, MD, the IHET Laboratory investigates the role of immunity, inflammation and epigenetics in various disease processes including tissue regeneration, diabetes, sepsis, aneurysm/atherosclerosis and other cardiovascular and cardiometabolic diseases. Our group (Frank Davis, MD and Andrea Obi, MD) is well-established in this translational space and works to advance our understanding of the basic mechanisms, pathways and proteins involved in regulating inflammation in tissues. The goal of this work, motivated in part by our groups’ vascular surgery clinical practices, is to identify new drug targets and therapies to improve clinical care in patients with cardiovascular and cardiometabolic disease.
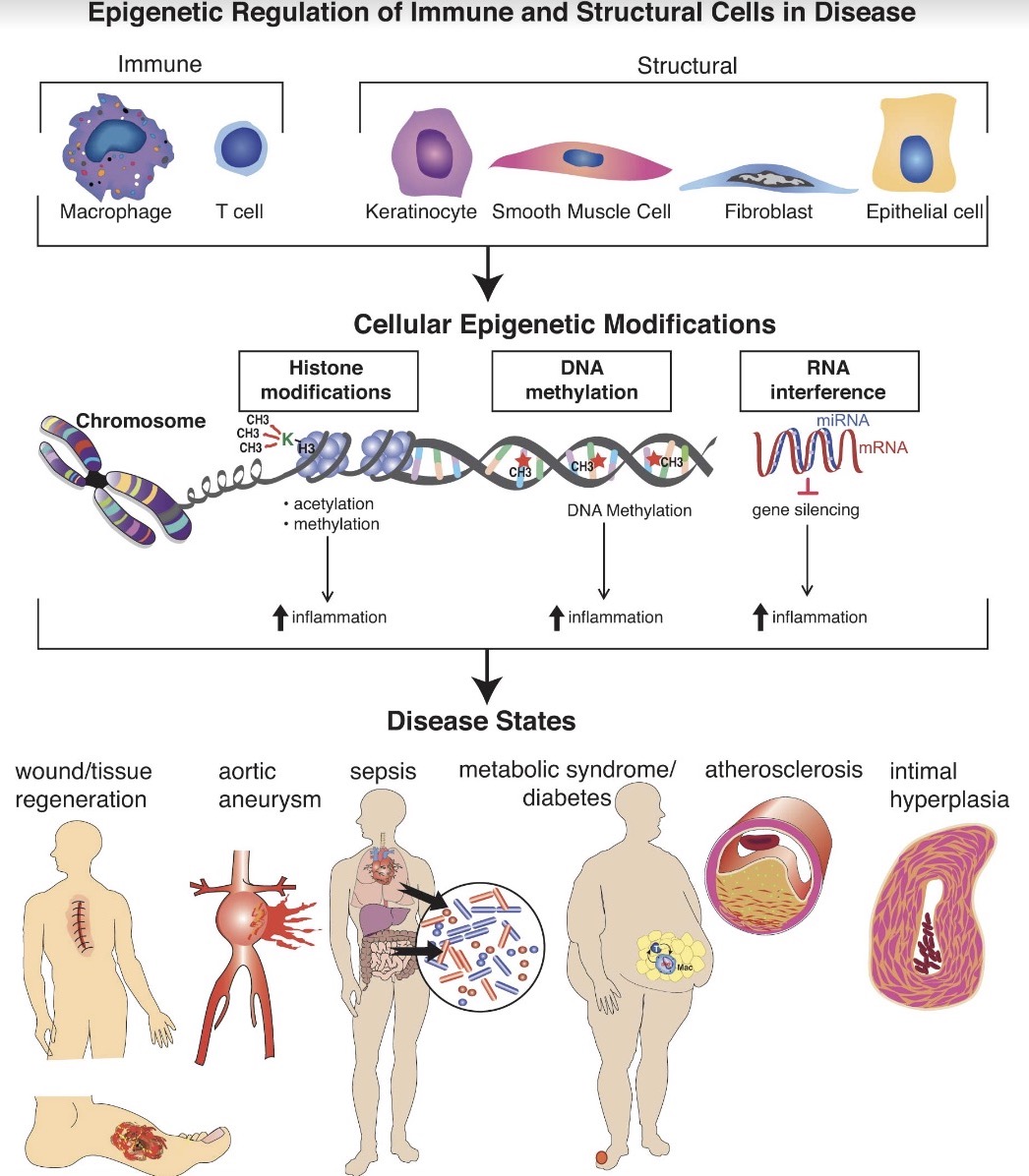
Epigenetic Regulation of Immune Cell Function in Disease
The main theme of our laboratory has been to examine how epigenetic alterations present in disease states can influence inflammatory gene transcription. This work has been broad and has examined various epigenetic enzymes including histone methyltransferases/demethylases (i.e., JMJD3, SETDB2, MLL1), DNA methyltransferases (i.e.,DNMTs) and microRNA (i.e., miR29), amongst others. We have examined upstream pathways that regulate these epigenetic enzymes including JAK/STAT, TLR4 and others. Our initial work focused on innate immune cells, namely macrophages, in various disease processes including tissue repair, diabetes, COVID19, sepsis and aneurysms; and now have expanded into atherosclerosis/intimal hyperplasia. We have also taken an interest in other structural and immune cells and how epigenetic alterations affect the cell’s function and plasticity (keratinocytes, fibroblasts, T cells, SMCs, ECs) in disease states. We have many ongoing exciting projects examining these themes and we continue to expand our interests based on our findings and the interests of the trainees.
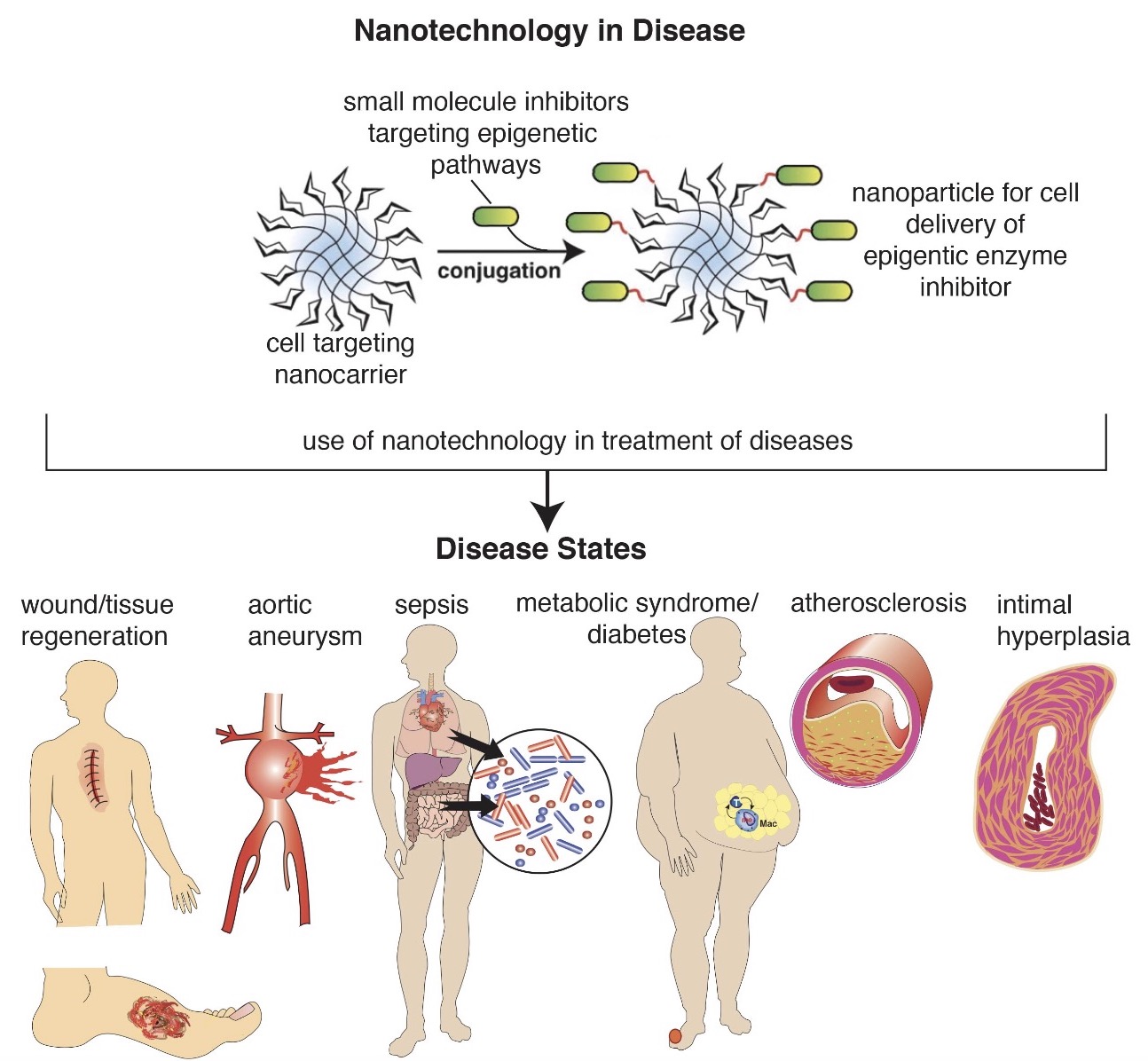
Nanotherapy Targeting Immune Signaling Pathways and Epigenetic Targets
The role of the immune system in cardiovascular health and disease is critical. In many cardiovascular pathologies, the immune system goes awry, causing dysregulated inflammation. Although there are many triggers for the immune system to become disinhibited, our lab focuses on epigenetic alterations in the immune cells that control their inflammatory potential. This allows us to target these mechanisms and along with our collaborators (Andrew Smith, PhD), design cell-and eventually patient specific therapies to restore the cells to their native state. The goal of the work in our laboratories is to identify therapeutic targets and develop effective new approaches so that we can better treat our patients.
Next Generation Sequencing
Given our translational focus, we have worked with our collaborators (Johann Gudjonnson, MD, PhD and Alex/Lam Tsoi, PhD) to examine human and murine tissues by single cell RNA sequencing, spatial sequencing, ATAC sequencing and more recently single nucleotide sequencing. We have integrated these datasets with GWAS data and examined key pathways mechanistically using murine models of pathology. We will continue to expand these sequencing projects in the coming years.